Researchers at Penn Develop Scar-like Culture Systems to Understand and Treat Fibrosis
A scar might be a reminder of an accident or surgery, but the fibrous tissue that makes up a scar also forms after a heart attack and arises in solid tumors as well as in chronic diseases such as liver cirrhosis and muscular dystrophy. Implanted medical devices and materials are similarly surrounded by fibrous capsules that impede their function.
Now, a study from the University of Pennsylvania has developed a ”‘scar in a dish” in order to uncover mechanisms that cells use to sense this fibrous tissue. The system will be helpful for understanding the roots of the scars’ stiffness, and ultimately lead to treatments that would help revert the tissue to its normal consistency.
Beyond healing scars, these treatments could be applied to diseases that range from heart failure to cancer.
The research was conducted by Dennis Discher, director of the new Physical Sciences in Oncology Center at Penn and the Robert D. Bent chaired professor in the Department of Chemical and Biomolecular Engineering in the School of Engineering and Applied Science, together with lead author and former graduate student P.C. Dave P. Dingal.
It was published in Nature Materials.
“In all sorts of diseases and with most types of major injury,” Discher said, “the body responds by walling off the damage with a collagen-rich patch. This scar can often help in the short term but can also cause long-term dysfunction of a tissue or organ. We want to understand and control how cells respond to scars in order to better control scarring in many contexts.”
Injections of certain types of adult-derived stem cells, known as mesenchymal stem cells, have been previously shown to help dampen scarring in animal models of injury. However, how the cells respond to scars and why they don’t do a better job in breaking down these walls has been a mystery.
To investigate, the researchers first analyzed fibrotic tissues and then made polymer-based gels to mimic their structure. Scarred tissues are complex but all have an abundance of stiff collagen fibers arranged in a fractal pattern, appearing roughly similar to how tree branches fill space. These fibers displace normal tissue and disrupt its function, and additional infiltrating cells contribute to overall changes in gene expression.
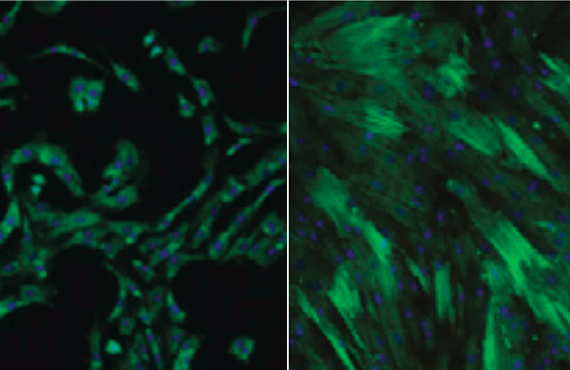
Some genes, such as smooth muscle actin, or SMA, are especially sensitive to scarring. Increased expression of SMA indicates at least some of the cells in the affected tissue are becoming bigger, stiffer and stronger, contributing to the scar.
The researchers aimed to use their simulated scar system to reveal how SMA is regulated, as its patches of varying stiffness would more fully stimulate these dynamics than the more homogeneous tissue models then available. Ultimately, understanding and manipulating SMA’s expression could be used to attenuate the damaging fibrotic tissue.
Reducing such complicated tissue to a simple but representative “scar in a dish” required choosing the appropriate cell type. Mesenchymal stem cells are a stem cell type found in related forms in many adult tissues, and they not only have some potential to differentiate into other tissue types but can also contribute to healing. These cells were already known to express SMA when cultured on rigid culture surfaces like the plastic dishes and glass coverslips that are widely used in biology labs.
“We found that SMA turns on slowly and stays on in these cells,” Dingal said, “but the scar-like polymer gels with heterogeneous stiffness gave surprisingly homogeneous expression of SMA when comparing different cells in the same culture. Based on gene expression profiling of scarred tissues and a couple of past studies, we guessed that SMA would somehow anti-correlate in our system with a transcription factor that could repress its gene expression: a protein called NKX2.5.”
NKX2.5 is known for its role in early development of heart, but evidence of its presence and function in these adult stem cells was lacking. Using new methods for measuring proteins by a method called mass spectrometry, together with several other standard approaches, NKX2.5 was found to shuttle between a cell’s nucleus and its cytoplasm, depending on the stiffness of the culture surface.
“Under the microscope,” Discher said, ”we could see that NKX2.5 was in the nucleus in cells on soft gels and outside the nucleus in cells on both stiff gels and the scar-like gels. Despite variations between different cells, the amount of SMA in a given cell related inversely in a switch-like fashion to the amount of NKX2.5 in the nucleus of the same cell.”
Similar observations were also made for embryonic heart cells, confirming that NKX2.5 is generally a mechanosensitive transcription factor.
“Our finding that both NKX2.5 and SMA show less cell-to-cell variation on the heterogeneous scar-like gels is likely due to the fact that stem cells crawl across collagen fiber bundles so that mechanosensitivity is dynamic, with NKX2.5 shuttling in-and-out of the nucleus,” Dingal said. “This tends to smooth out extreme responses that become locked in on rigid substrates.”
The level of NKX2.5’s expression was then controlled together with its localization to either the nucleus or the cytoplasm. Using these molecular biology methods, SMA levels went down as NKX2.5 levels went up, leading the authors to conclude that NKX2.5 is a mechanosensitive repressor of SMA.
Importantly, long-term cultures of the stem cells on rigid plastic led to degradation of NKX2.5 so that there was even less in the nucleus. SMA was predictably and slowly accumulating in these cells, consistent with the long-lasting nature of scars.
Such findings can help explain why injection of these types of stem cells into stiff scars led to limited improvement of fibrosis.
The findings also show that engineering these cells to achieve high nuclear levels of NKX2.5 can maintain a cell phenotype matched better to normal, soft tissue. Such cells could be better for regenerative therapies.
The researchers provide additional examples for how their scar-like cultures can be used to discover many other molecules and pathways that could be manipulated to better treat fibrosis in many disease contexts, from skin scars to heart and muscle disease as well as some cancers.
Additional members of the Discher lab who contributed to the study included Andrew M. Bradshaw, Sangkyun Cho, Matthew Raab, Amnon Buxboim and Joe Swift.
The research was supported by the National Cancer Institute’s Physical Sciences in Oncology Centers program, the American Heart Association, National Heart Lung and Blood Institute of the National Institutes of Health, the U.S.-Israel Binational Science Foundation and the National Science Foundation, including Penn’s Materials Research Science and Engineering Center and Nano/Bio Interface Center.