Penn Study Describes the Molecular Cause of Common Cerebrovascular Disease
Cerebral cavernous malformations (CCMs) are clusters of dilated, thin-walled blood vessels in the brain that can cause stroke and seizures, yet exactly how they form is somewhat of a mystery. Now, a team from the Perelman School of Medicine at the University of Pennsylvania has discovered the molecular mechanism that underlies this common cerebrovascular disease. They published their results this week online ahead of print in Nature.
CCM disease, which occurs in about one in 100 to 200 people, can present in two forms. One is sporadic, accounting for 80 percent of cases, and is most frequent in older individuals. The remaining 20 percent are familial, inherited cases. These patients present with a large number of lesions and more severe symptoms that arise in much younger individuals. CCM disease is a progressive disorder, and the standard of care remains surgical removal of the most dangerous lesions and symptom management.
When CCM was first identified as a genetic disease, mutations in one of two copies of three genes were identified in human patients. The proteins encoded by these genes were found to bind each other in a single complex, but this complex lacks intrinsic enzyme activity and how its loss results in vascular disease - and why this vascular disease arises so specifically in the brain – has remained unknown.
“Despite all we know about CCMs, how loss of the CCM complex causes disease remains controversial, with numerous downstream signaling pathways and processes proposed to play roles,” said senior author Mark Kahn, MD, the Edward S. Cooper, MD/Norman Roosevelt and Elizabeth Meriwether McLure Professor in the department of Medicine. An important clue to disease pathogenesis was discovered while studying vertebrate heart development, when the Kahn lab found that CCM proteins in endothelial cells control the activity of the enzyme MEKK3 and a downstream gene expression program that is essential for the embryonic heart to properly develop.
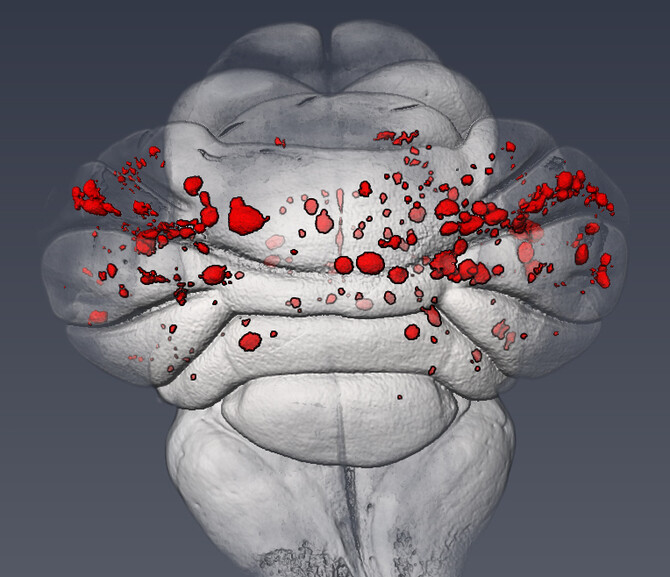
For the Nature study, the Kahn lab investigated whether a conserved mechanism might also underlie the formation of CCM lesions in the postnatal brain. Using a neonatal mouse model of CCM disease, they found that expression of MEKK3 target genes, KLF2 and KLF4, was increased in the cells of newly formed CCM lesions. “From there we were able to demonstrate that partial, endothelial-specific loss of the genes for MEKK3, KLF2, or KLF4 completely rescues lesion formation and prevents the mice from dying due to CCM disease,” Kahn said.