Penn Medicine: Genetic Variation Determines Protein’s Response to Anti-diabetic Drug
In the first study of its kind, Penn researchers have shown how an anti-diabetic drug can have variable effects depending on small natural differences in DNA sequence between individuals. Mitchell Lazar, MD, PhD, Raymond Soccio, MD, PhD, and colleagues at the Perelman School of Medicine at the University of Pennsylvania, aim to apply this knowledge to develop personalized approaches to treating diabetes and other metabolic disorders. The team published their findings this week in Cell.
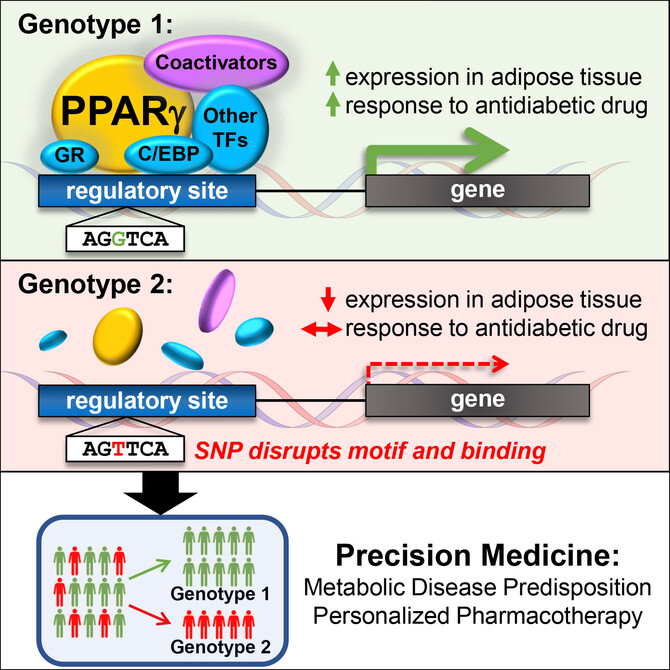
Lazar and team focused on the important fat cell molecule called PPAR-gamma, which is the target of the thiazolidinedione (TZD) class of drugs used to treat type 2 diabetes. PPAR-gamma binds to DNA at switches that turn other genes on, and the Penn researchers showed that natural genetic differences in the DNA of this regulatory switch could determine whether PPAR-gamma and TZD drugs could turn other genes on. “The implications of this work go beyond PPAR-gamma and TZDs, to all drug targets that function directly at the genome to regulate physiology in health and disease,” said Soccio, the study’s lead author and an instructor in Medicine. Indeed, 20 percent of all prescriptions written are for such drugs as thyroid hormone and steroids that target nuclear receptor proteins related to PPAR-gamma, noted senior author Lazar, professor of Medicine & Genetics and director of the Institute for Diabetes, Obesity, and Metabolism.
The genetic differences are called SNPs, or single nucleotide polymorphisms, and are variants in the DNA alphabet of A, T, C, and G molecules that occur naturally among individuals. Many such SNPs have been associated with disease risk, for instance showing that a person with an A at a given location in DNA has a higher risk of diabetes compared with someone with a G. However, these disease-related SNPs often reside in the so-called “dark matter” of the genome that does not directly code for genes, but includes those switches that control genes.
Lazar and his team showed that SNPs in PPAR-gamma switches provide a mechanism for the disease-risk associations. For example, one such SNP was linked to blood lipids, including HDL (“good” cholesterol) and triglycerides; type 2 diabetes; high blood pressure; and waist-hip ratio (a measure of “apple” versus “pear” body shape in obesity). This constellation of findings is called “the metabolic syndrome”, and Soccio points out that “it’s remarkable that a single change in a DNA letter determines whether PPAR-gamma binds to one regulatory site in fat tissue, and this may alter a person’s risk of metabolic syndrome.”
Click here to view the full release.