Penn Medicine: Adding to the List of Disease-Causing Proteins in Brain Disorders
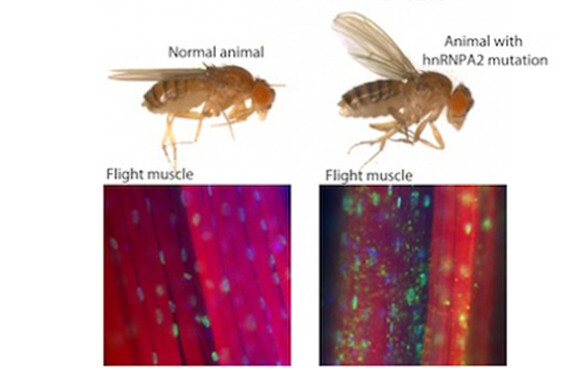
PHILADELPHIA — A multi-institution group of researchers has found new candidate disease proteins for neurodegenerative disorders. James Shorter, Ph.D., assistant professor of Biochemistry and Biophysics at the Perelman School of Medicine, University of Pennsylvania, Paul Taylor, M.D., PhD, St. Jude Children’s Hospital, and colleagues describe in an advanced online publication of Nature that mutations in prion-like segments of two RNA-binding proteins are associated with a rare inherited degeneration disorder affecting muscle, brain, motor neurons and bone (called multisystem proteinopathy) and one case of the familial form of amyotrophic lateral sclerosis (ALS).
“This study uses a variety of scientific approaches to provide powerful evidence that unregulated polymerization of proteins involved in RNA metabolism may contribute to ALS and related diseases,” said Amelie Gubitz, Ph.D., a program director at the National Institute of Neurological Disorders and Stroke (NINDS).
ALS, or Lou Gehrig's disease, is a universally fatal neurodegenerative disease. Previous studies found that mutations in two related RNA-binding proteins, TDP-43 and FUS, cause some forms of ALS, but more proteins were suspected of causing other forms of the disease. TDP-43 and FUS regulate how the genetic code is translated for the assembly of proteins.
There are over 200 human RNA-binding proteins, including FUS and TDP-43, raising the possibility that additional RNA-binding proteins might contribute to ALS pathology. Computer algorithms, based on protein sequences, designed to identify yeast prions predict that around 250 human proteins, including several RNA-binding proteins associated with neurodegenerative disease, harbor a distinctive prion-like segment. These segments are essential for the assembly of certain protein complexes. But, the interplay between human prion-like segments and disease is not well understood.
Using yeast as a model organism, co-author Aaron Gitler, while at Penn in 2011, surveyed 133 of 200-plus candidate human RNA-binding proteins to predict new ALS disease genes, other than TDP-43 and FUS. They further winnowed the candidates to about 10 proteins with prion-like segments, and selected two candidates, TAF15 and EWSR1, for further study. Both TAF15 and EWSR1 aggregated in the test tube and were toxic in yeast.
Remarkably, they also uncovered TAF15 and EWSR1 mutations in ALS patients that were not found in healthy individuals. Based on these findings, they proposed that RNA-binding proteins with prion-like segments might contribute very broadly to the pathology of ALS and related brain disorders.
Click here to view the full release.