Penn Chemists Put a New Twist on Chirality
Biological molecules are chiral. Like gloves, they have either left- or right-handed versions that can’t be superimposed on one another. Macromolecules like DNA are also chiral and are exclusively made of building blocks with the same handedness.
Because of their ubiquity, it has been long supposed that this “homochirality” is a prerequisite for life, but research led by University of Pennsylvania chemists is calling this assumption into question.
A new study has discovered a class of molecules that can produce a double helix more consistent and more highly ordered than that of DNA, despite being made of a random mix of left- and right-handed building blocks.
The ability to form such structures out of heterochiral parts also has implications for industrial chemistry, opening doors to simpler techniques of making stable plastics, new ways of storing data and better kinds of photovoltaic cells and light-emitting diodes.
The research was led by Virgil Percec, the P. Roy Vagelos Professor of Chemistry in Penn’s School of Arts & Sciences. Also contributing to the study were members of Percec’s lab: Cécile Roche, Hao-Jan Sun, Pawaret Leowanawat, Benjamin E. Partridge, Mihai Peterca, Daniela A. Wilson and Margaret E. Prendergast, as well as Paul Heiney, professor in Penn’s Department of Physics and Astronomy.
They collaborated with researchers from the RIKEN Center for Emergent Matter Science in Wako, Japan; the Max-Planck Institute for Polymer Research in Mainz, Germany; and the University of Sheffield in the United Kingdom.
It was published in Nature Chemistry.
Every strand of DNA on Earth is right-handed, with its clockwise double helix composed of right-handed nucleotides. Sugars and amino acids are similarly uniform in their chiral composition. Life elsewhere in the universe might very well make use of the left-handed versions of these molecules, but they would be incompatible with their mirror images. All known biological interactions depend on matched chirality for functional groups and receptors to align properly.
The chiral uniformity of the molecules of life extends down to the parts from which they are made, a quality known as homochirality.
“Does life require homochirality to accomplish the same biological structures? What we’re suggesting in this paper is that the answer is no,” said Percec. “There is a mechanism by which you can build these structures from molecules with any kind of mix of handedness.”
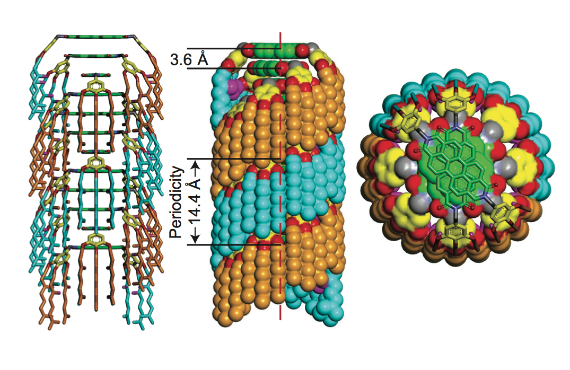
The key to this mechanism is selecting molecules whose stereocenters, the parts that give them either left- or right-handedness, are in a certain place when assembling into macromolecules.
“We call the mechanism a ‘cogwheel,’” said Percec. “The molecules self-assemble to form cogwheel-like columns. The stereocenters are in the inner part of the columns, while the ‘teeth’ on the exterior of the column enable the columns to interlock to form highly ordered crystals. There’s a large diversity of molecules that will follow this principle.”
The researchers demonstrated the ability of such molecules to produce highly regular and stable helical columns despite different degrees of heterochirality, confirming their structures with X-ray diffraction and spectroscopy.
Because of their cogwheel shape, columns with different handedness can interlock into even larger molecule assemblies.
“Because the chiral center points inward into the column,” Partridge said, “the exterior of the column looks identical, regardless of the chirality of the building blocks. So, a right-handed helix made of only right-handed molecules looks the same as a right-handed helix made of a mixture of right- and left-handed molecules. The columns of the same handedness then pack together just as well, regardless of the mix of chiral molecules used to make them.”
Beyond biological molecules, many synthetic molecules derive their structural properties from homochirality. The stiffness of polypropylene, a common plastic polymer, varies significantly based on the uniformity of its chiral building blocks.
“When polypropylene is roughly 90 percent homochiral or more, it becomes crystalline, so you can make things like carpets and chairs out of it. But, if it’s heterochiral, it has the consistency of ear wax,” Percec said.
Being able to achieve polymers with high stability and melting points without having to select only left- or right-handed molecules would simplify many manufacturing processes.
Highly crystalline materials are also desirable for applications in photovoltaics or organic electronics but usually require homochiral building blocks. This work opens the door for highly crystalline materials made from a mix of chiral building blocks, which are much less expensive and easier to synthesize.
The research was supported by the National Science Foundation, Humboldt Foundation, U.K. Engineering and Physical Sciences Research Council and Howard Hughes Medical Institute.